Research Interests
My current research interests at Marshall University stem from my graduate student research experiences obtained at Kansas State University and my postdoctoral experiences at the University of Chicago; these interests include nanoparticle (NP) synthesis, NP super-lattice formation via self-assembly at liquid interfaces, applications of NP self-assembled membranes, and the study of colloids at interfaces. Recent research has primarily focused on using azo dye functionalized polycarbonate membranes for water filtration applications. If interested, see recent peer reviewed work, Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal, published in the journal Micromachines. Previous peer reviewed works published on the synthesis and self-assembly of various 5-10 nm gold core nanoparticles to make nanoparticle-based structures for mechanical strength testing and water filtration applications [Patent No. - WO2013074669 - Nanoparticle-Based Desalination and Filtration System] and studying the line tension parameter of particles at liquid vapor interfaces are available in: Nature Communications & Progress in Surface Science, specifically section [3.1], respectively.
What is happening in the above figure?
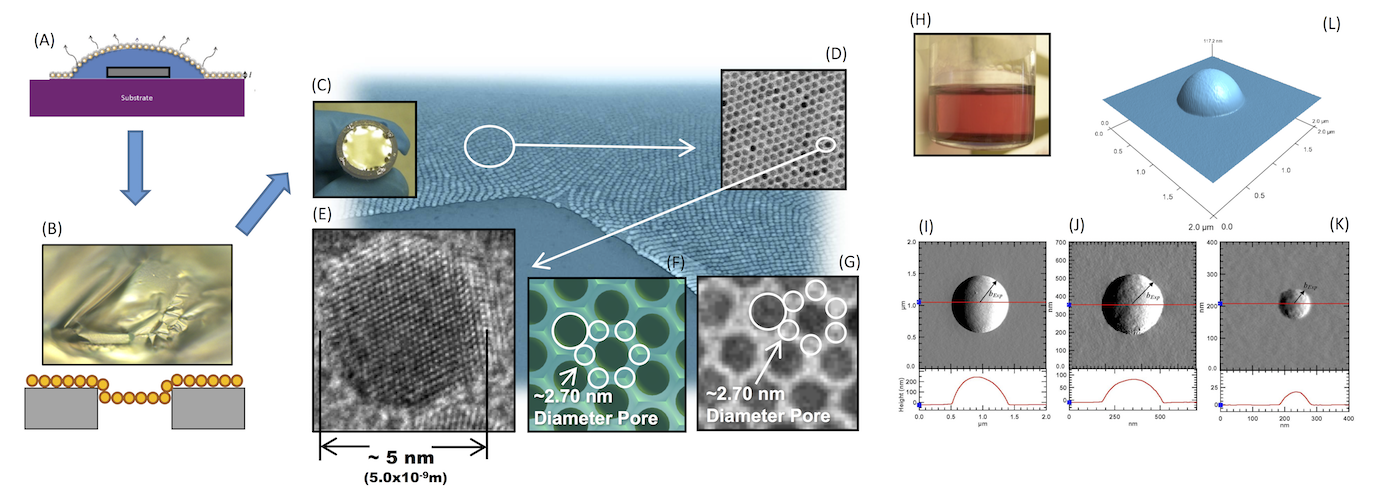
Optimial viewing for the above image is on a 21 inch wide screen. Click here to see the full image if using a device with a smaller screen.This figure is a collage of images that make up the different facets of my research interests. (A-C) This series of images depicts how self-assembled gold NP membranes are formed and how they are made to cover the openings of a porous substrate. The porous substrate is represented by the grey disk in figure (A), which is completely immersed in ultra-high purity water shown in blue. The ~5 nm diameter gold NPs, which are distributed in a water-insoluble organic solvent, are then deposited at the three phase contact line between the water, vapor, and solid. Self-assembly then occurs on the water interface as the solvent evaporates. The NP monolayer than slowly deposits itself onto the porous substrate as the water evaporates. (B) Shows a ~ 100 x magnification optical image of the NP membrane as the water slowly evaporates. Eventually, the membranes recede into the pores as the sample completely dries. (C) Shows the finished product prior to assembly in a filter housing. (D, E, G, and the blue background image) Show Transmission Electron Microscopy (TEM) images of the gold NPs at different magnifications. Collaborators at the University of Chicago took the high-resolution TEM image (E), while I took the other images at Argonne National Laboratory. (F) Shows a top view of a 3D rendered model, demonstrating that a ~ 2.7 nm diameter pore potentially exists between the gold core NPs; (G) shows that this 2.7 nm pore is in good agreement with experimental results. (H) Is a picture of what the gold NP solution looks like prior to depositing a small amount at the three phase contact line. Images (I-L) move away from looking at NP membranes, but instead focuses on NPs at lquid-vapor interfaces. Images (I-L) show different Atomic Force Microscopy (AFM) images of NPs protruding from a frozen liquid interface. From the final equilibrium geometry of the particle at the liquid-vapor interface, which is captured in the AFM images, the line tension parameter of the system can be determined; the line tension parameter plays a role in the stability of particles at these types of interfaces.
Students, are you interested in nanoscience or the above projects?
If you are interested in nanoscience, or the projects discussed here, please drop by my office in the Science Building, Room 152 at any time, or email me. We can discuss the possibilities of you starting a summer/semester research project, a capstone project, or a more long-term project over the more senior years of your undergraduate career. If you are a freshmen, come and see me. I can give you papers to read, and then you can find more information and more related papers on your own in between your classes. Then we can discuss in more detail what type of project you want to do in the future; if you are doing well in your classes, we will implement these discussed ideas for projects in your sophomore year. Independent of you being a freshman, a sophomore, a junior, a senior, or a graduate student, come and see me, or send me an email, this is the first step to get working in a research laboratory. Keep in mind, before you can become an excellent undergraduate researcher, you must first become an excellent student in the classroom, understanding the fundamentals of science in the world around you. Therefore, at this point as an undergraduate student, you must always put your education and classwork as a first priority next to experimental research in the laboratory. Understand as a college student, you should check your @marshall.edu email twice a day at a minimum. Many exciting and important oppurtunities are lost by students who simply do not check and respond to emails. Checking your email frequently, minimum of twice a day, is a requirement to work in this research lab.
-
For details regarding Dr. Sean P. McBride's education & appointment history click here.
For a full list of downloadable versions of available publications click here.
Selected Publications
(2022) Micromachines, 13(4), 577. “Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal.” Carrie Cockerham, Ashton Caruthers, Jeremy McCloud, Laura M. Fortner, Sungmin Youn, and Sean P. McBride (Marshall University).
(2018) Soft Matter, 14, 9107-9117. “Conforming nanoparticle sheets to surfaces with Gaussian curvature.” Noah P. Mitchell, Remington L. Carey, Jelani Hannah, Yifan Wang, Maria Cortes Ruiz, Sean P. McBride (Marshall University), Xiao-Min Lin, and Heinrich M. Jaeger.
(2017) ACS Nano, 11, 8, 8026-8033. “Thermo-Mechanical Response of Self-Assembled Nanoparticle Membranes.” Yifan Wang, Henry Chan, Badri Narayanan, Sean P. McBride (Marshall University), Subramanian K. R. S. Sankaranarayanan, Xiao-Min Lin, and Heinrich M. Jaeger.
(2017) Progress in Surface Science (Review Article), 92 (1), 1-39. “Line tension and its influence on droplets and particles at surfaces.” Bruce M Law, Sean P. McBride (Marshall University), Jiang Wang, Haeng Sub Wi, Govind Paneru, Santigo Betelu, Bret Flanders, Hiroko Matsubara, Takanori Takiue, and Makoto Aratono.
(2016) Nanotechnology, 27 (28), 285301. “Hybrid nanostructures of well-organized arrays of colloidal quantum dots and a self-assembled monolayer of gold nanoparticles for enhanced fluorescence.” Xiaoying Liu, Sean P. McBride (Marshall University), Heinrich M. Jaeger, and Paul F. Nealey.
(2015) Nano Letters, 15 (10), pp 6732–6737. “Strong Resistance to Bending Observed for nanoparticle Membranes.” Yifan Wang, Jianhui Liao,Sean P. McBride, Efi Efrati, Xiao-Min Lin, and Heinrich M. Jaeger.
(2015) Faraday Discussions, 181, 365-381. “Properties of self-assembled nanostructures: general discussion.” Javier Reguera, et. al.
(2015) Faraday Discussions, 181, 325-338. “Mechanical properties of self-assembled nanoparticle membranes: bending and stretching.” Yifan Wang, Pongsakorn Kanjanaboos, Sean P. McBride, Edward Barry, Xiao-Min Lin, and Heinrich M. Jaeger.
(2014) Nature Communications, 5, Article Number: 5847. "Ion Transport Controlled by Nanoparticle-Functionalized Membranes." Edward Barry, Sean McBride, Heinrich Jaeger, and Xiao-Min Lin.
Image Gallery
Click Here For Previous Image.